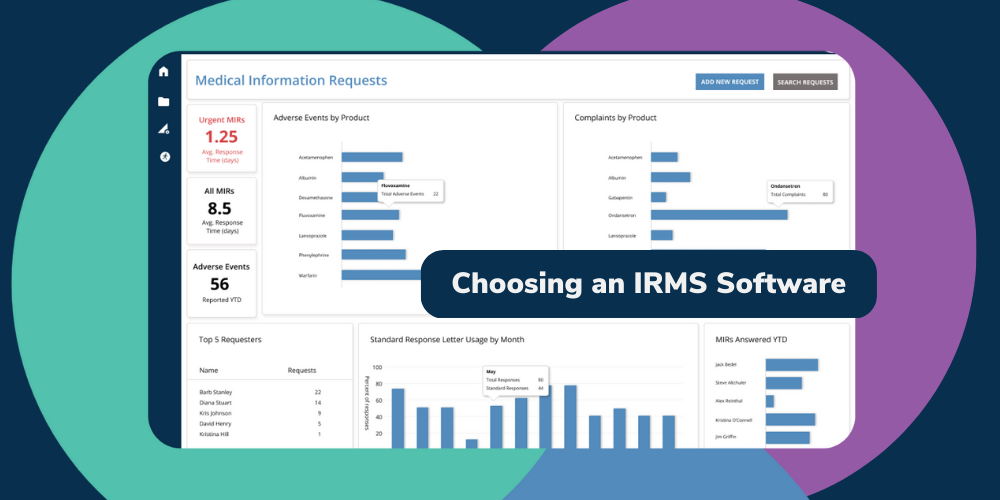
Medical information requests (MIRs) are a crucial part of the healthcare and life sciences industry, allowing healthcare providers to obtain the information they need to provide the best possible care for their patients. However, without an efficient MIR management process, delays and inaccuracies can jeopardize patient safety, regulatory compliance, and a company's reputation.
In this guide, we'll explain what MIRs are, why they matter, and share best practices for handling them. We'll also cover the benefits of using a centralized management strategy and process automation tools, such as BP Logix's MIR solution, which can alleviate common MIR management challenges and ensure prompt, accurate, and compliant handling.
Whether you're a medical affairs professional, healthcare provider, or pharma executive, this guide will help you navigate the complex world of medical information requests. Already well-versed in MIRs? Skip ahead to MIR management best practices!
Types of medical information requests
MIRs can take many forms:
Let's delve into what these MIR types entail and what role they play in the lives of healthcare providers, patients, and medical affairs teams.
Adverse event reporting
Adverse event reporting is a critical component of medical information requests. In this type of request, healthcare providers submit information about any undesirable or unexpected health events experienced by patients while using a particular medication or medical device. These events could range from mild side effects to severe allergic reactions or even fatalities.
For instance, imagine a patient is prescribed a new medication and then experiences an adverse health event that they suspect is caused by the medication. The healthcare provider would then create an adverse event report to submit to the company that manufactures the medication. This report would contain detailed information about the patient, the medication, and the adverse event experienced.
Pharmaceutical companies must carefully track and promptly respond to all adverse event reports they receive. Failure to do so can have severe consequences, including patient harm, regulatory sanctions, and reputational damage. Effective management of adverse event reporting is essential for maintaining patient safety and ensuring that products remain compliant with regulatory requirements.
Product information requests
Product Information Requests (PIRs) are a common type of MIR that medical affairs teams handle. PIRs seek general information about a product such as its indications, dosages, contraindications, and clinical trial results.
For example, a physician might request PIRs to understand whether a medication would be a suitable option for their patient with a specific condition. Another provider may want more information on a medication than what is listed on the label.
Handling PIRs requires the ability to provide comprehensive information about the product to healthcare providers while ensuring regulatory compliance. It is essential to handle these requests efficiently as they could impact providers’ decision-making process and, ultimately, patient outcomes.
Medical inquiries
Medical inquiries are a specific type of medical information request that requires a detailed response from a medical affairs team. These requests are often initiated by healthcare providers seeking guidance on a specific medical issue or question related to a product.
Let's say a provider inquires about the appropriate dosage for a patient with a specific medical condition, or the potential drug interactions of a product with other medications. As with other types of MIRs, it is important for companies to respond to medical inquiries in a timely and accurate manner to provide healthcare providers with the necessary information to make informed decisions.
Clinical study data
Clinical study data requests are another common type of MIR. Healthcare providers may request access to detailed clinical study data, such as results from clinical trials, to help them make informed decisions about treatment options for their patients.
For instance, a physician may request clinical study data on a new medication before prescribing it to their patient. This request may include information about the drug’s safety, efficacy, and potential side effects.
Handling clinical study data requests requires companies to provide accurate and comprehensive data while adhering to regulatory requirements for data privacy and protection.
Off-label information
Off-label information requests are a specific type of MIR that requires companies to respond to requests for information about the use of a product outside of its approved label indications. These requests could include information about the potential benefits and risks of using a product off-label.
Let's say a healthcare provider inquires about using a medication to treat a condition not listed on its label indications. In these cases, companies must ensure that their responses are accurate and do not promote the off-label use of their product.
Handling off-label information requests requires companies to carefully evaluate the information they provide and ensure that their responses comply with regulatory requirements.
Product complaints
Product complaints are a type of MIR that companies use to collect and address customers' concerns about their products. These complaints can range from product quality to issues with packaging, labeling, or other components of the product.
Imagine a patient files a complaint about a medication they took because they believe it's causing them harm. A company must investigate the complaint thoroughly and respond in a timely manner to address any issues with their product.
Handling product complaints requires companies to have an effective complaint management process in place to investigate and address concerns raised by patients.
Why medical information requests matter
MIRs have a significant impact on providers and patients, as well as medical affairs professionals and the organizations they represent. As such, it is essential for life science companies to establish clear MIR submission protocols and develop an efficient system for processing incoming requests.
While larger companies often have a dedicated team to handle MIRs, smaller organizations may have limited resources and rely on a smaller team to manage these requests. Regardless of the size of the organization, it is crucial to handle MIRs efficiently and respond to them promptly in order to:
Let's take a closer look at why mastering medical information request management is vital in these areas:
Regulatory compliance
Pharmaceutical and medical device companies have a legal obligation to provide timely, accurate information about their products to the general public, patients, and healthcare professionals. Failure to comply can result in severe consequences, including legal actions, fines, and damage to your company's reputation.
The Food and Drug Administration (FDA) mandates this reporting and outlines pharmaceutical companies' responsibilities in its authoritative guide, “Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices.”
Under these guidelines, pharmaceutical companies must respond truthfully to unsolicited medical information requests. Misleading information and off-label promotion of product uses are prohibited in these responses, and the company must maintain detailed records of all MIRs and their responses.
Regulatory requirements for MIRs are becoming increasingly stringent. Non-compliant organizations risk penalties and other punitive measures. By ensuring regulatory compliance, you protect your business continuity and profitability.
Patient safety
Prompt and accurate responses to medical information requests are crucial to ensuring patient safety. When healthcare providers have access to the information they need about medications and devices, they can make informed decisions that promote positive health outcomes.
However, when pharmaceutical companies are slow to respond to MIRs, patient safety can be compromised. Providers may be hesitant to prescribe a particular medication, potentially resulting in delayed or inadequate treatment for patients.
MIRs also provide healthcare providers with a way to report concerns or adverse events, which pharmaceutical companies can use to improve products and protect patients from the risks associated with faulty devices or medications. By prioritizing the efficient management of MIRs, companies can maintain a strong focus on patient safety and improve overall healthcare outcomes.
Reputation management
In today's age of social media and online reviews, negative news can spread rapidly, potentially harming a pharmaceutical company's reputation. To stay ahead of any rumors or concerns, it's crucial to respond quickly and effectively to medical information requests.
Swift, accurate responses to MIRs can help put out any potential fires before they spread too far. This proactive approach can help maintain public trust and protect a company's reputation from the fallout of adverse events.
On the other hand, slow or inaccurate responses can have the opposite effect, damaging public trust and causing patients to seek out competitor products. Mishandling MIRs can ultimately lead to decreased product usage and revenue loss.
Competitive advantage
In the fiercely competitive industry of life sciences, companies must go the extra mile to maintain an edge over their rivals. Responding to medical information requests promptly is an excellent way for companies to gain a competitive advantage.
A streamlined MIR process shows healthcare providers that a company is transparent, trustworthy, and patient-focused. These factors are crucial in building long-term trust and relationships with providers and patients.
By developing an efficient MIR process, pharmaceutical companies can help providers make informed decisions about prescribing their products. This proactive approach can alleviate prescription hesitancy and increase the likelihood of a provider choosing their product over a competitor's.
Ultimately, having an efficient and effective MIR process can set a company apart from its rivals and contribute to its long-term success.
Continuous improvement
In order to provide better outcomes for patients, life science organizations must be able to stay ahead of the curve. This is another area in which MIRs shine — they can provide valuable insights that may help companies innovate and drive continuous improvement in their products.
For example, imagine there is a medication indicated for pain relief that could also help patients overcome sleep issues. By bringing this information to organizational decision-makers, the company could obtain funding to explore other uses for the medication — ultimately helping them stay competitive in the market, generate more revenue, and improve patient outcomes.
Best practices for handling medical information requests
Managing medical information requests can be challenging, but life science companies can simplify the process and promote transparency by following a few established best practices.
Specifically, organizations tasked with handling medical information requests should:
1. Develop a clear, standardized process
The first step to mastering medical information request management is developing a clear and standardized process.
A well-designed process will act as a blueprint for the medical information team to follow and should cover the intake, response, tracking, and record-keeping aspects of MIR. It should also align with FDA guidelines to guarantee compliance and prevent any monetary repercussions.
Without a clear process to follow, the medical information team may miss key steps in the process, leading to delays in responding to requests, inaccurate information, and even noncompliance with FDA regulations. Inconsistent processes can also lead to confusion among team members, making it difficult to track and manage requests efficiently. A standardized process is essential for ensuring that all medical information requests are handled promptly, accurately, and in compliance with regulations.
To create a robust and comprehensive process, it's essential for decision-makers to gather feedback and input from the people who are most familiar with the day-to-day management of medical information requests. This feedback can help ensure that the process is clear, comprehensive, and easy to follow for all team members. The end result is a well-designed process that meets the needs of the organization while also ensuring compliance and efficiency in managing MIRs.
2. Create a dedicated medical information team
All organizations, regardless of size, should establish a dedicated medical information team to manage MIRs. This will ensure that requests are promptly and efficiently processed, and nothing falls through the cracks. If responsibilities are delegated to other teams, such as sales or marketing, it can lead to delayed responses, compliance issues, and damage to a company's reputation.
Creating a dedicated MIR team will require effort, but the benefits will be worthwhile. This team will be better equipped to handle requests, maintain compliance, and ensure that information is communicated transparently.
While some smaller companies may not have the resources to create a dedicated medical information team, there are ways to overcome this challenge and still manage MIRs effectively.
One option is to assign MIR responsibilities to a small group of individuals within the organization who have transferrable expertise and knowledge to handle these responsibilities.
Another option is to harness process automation to help streamline MIR management and reduce the need for a large team. Automated systems can be used to track and manage requests, respond to routine inquiries, and flag urgent requests for immediate attention. This can help smaller organizations manage MIRs more efficiently while maintaining compliance and ensuring prompt responses to healthcare professionals.
3. Use a centralized system to manage MIRs
Managing medical information requests through email or shared files can be risky and cause unnecessary confusion. Without a centralized system to manage MIRs, time-sensitive requests can get lost or overlooked, leading to delayed responses and a risk of noncompliance. This can cause friction among healthcare providers and ultimately damage the organization's reputation.
Additionally, managing MIRs without a standardized process can create confusion among team members about their roles and responsibilities, leading to inconsistent responses and inaccurate information being disseminated to healthcare providers.
Implementing a centralized system to manage MIRs will simplify the process, prevent any mishaps, and ensure that all requests are responded to promptly. Storing MIR data in a single location will help the MIR team leader oversee the management of requests and verify that the team responds to them appropriately based on their priority and urgency.
4. Implement a prioritization system
Pharmaceutical companies should prioritize MIRs to ensure that urgent requests are handled before routine inquiries. Developing a prioritization system will help the medical information team manage their workload and address requests based on their urgency.
For instance, consider a medical information team that receives two MIRs on the same day: one is a routine inquiry about dosage information, while the other reports a potential adverse event associated with one of the company's products. In this scenario, the team should prioritize the adverse event MIR, addressing it promptly to mitigate potential risks to patient safety. MIRs that are of equal urgency should be handled based on the order they are received.
Having a clear prioritization system in place ensures that the team is efficiently managing their workload while addressing the most pressing issues first.
5. Monitor MIR response performance
Want to ensure that your medical information request management program is continuously improving? Consider monitoring the the performance of your organization's MIR responses. You can gain valuable insights to make data-driven decisions by tracking metrics like response times, request volumes, and other relevant data points.
For example, let's say a pharmaceutical company notices an increasing trend of requests regarding potential off-label uses of their product. By monitoring this trend and analyzing the data, the company can conduct further research into the potential off-label uses, test the product's efficacy in those areas, and potentially apply for FDA approval for new indications.
This type of data-driven decision-making leads to product innovation, new opportunities in the market, and increased revenue.
On the other hand, neglecting to monitor MIR response performance could lead to missed opportunities for growth and potential noncompliance with regulatory requirements. For example, let's say a pharmaceutical company receives an MIR regarding the use of their product in a specific patient population. Monitoring MIRs may help the company identify potential gaps in how they are labeling or indicating their product. Failing to address these gaps puts the company at risk for being noncompliant with FDA regulations, resulting in fines, penalties, and PR nightmares.
By monitoring MIR response performance, companies can avoid regulatory issues and protect their reputation from negative PR. The insights gained from monitoring response performance can also help companies continuously improve their MIR process as a whole by allocating resources more effectively and going the extra mile to meet the needs of healthcare providers and their patients.
Automating your centralized MIR system
Implementing the best practices and centralizing your MIR system is essential for compliance and efficient management of medical information requests. However, not all centralized systems are equal, and rudimentary ones such as shared email inboxes lack the tools and management capabilities necessary to ensure compliance.
To meet FDA requirements for adverse event handling and auditing, it's crucial to route inquiries to the appropriate team members. Failing to do so can result in difficulty obtaining treatment approvals and even revocation of previously approved treatments, as demonstrated by the cases of chloroquine phosphate and hydroxychloroquine sulfate.
 To ensure compliance and efficient MIR management, consider implementing a powerful management system that includes sophisticated process automation tools. Leveraging tools such as BP Logix’s MIR solution enables you to tap into the power of predictive analytics, facilitate centralized data management, automate workflows, and integrate your database with existing systems.
To ensure compliance and efficient MIR management, consider implementing a powerful management system that includes sophisticated process automation tools. Leveraging tools such as BP Logix’s MIR solution enables you to tap into the power of predictive analytics, facilitate centralized data management, automate workflows, and integrate your database with existing systems.
Process automation tools not only streamline your MIR system but also reduce manual errors, speed up response times, and enable proactive management of requests, leading to a more efficient and compliant system.
Optimize MIR management with BP Logix
Medical information requests are a critical aspect of the healthcare industry, with the potential to influence patient outcomes, regulatory compliance, and revenue generation. As such, it's imperative to have a robust and streamlined process in place to manage them effectively.
By embracing process automation and optimization tools, you can revolutionize your approach to MIRs and achieve a more efficient and compliant management system. With BP Logix's MIR solution, you can leverage cutting-edge predictive analytics, centralized data management, and customizable workflow automation to streamline your MIR process and reduce the risk of errors and compliance issues.
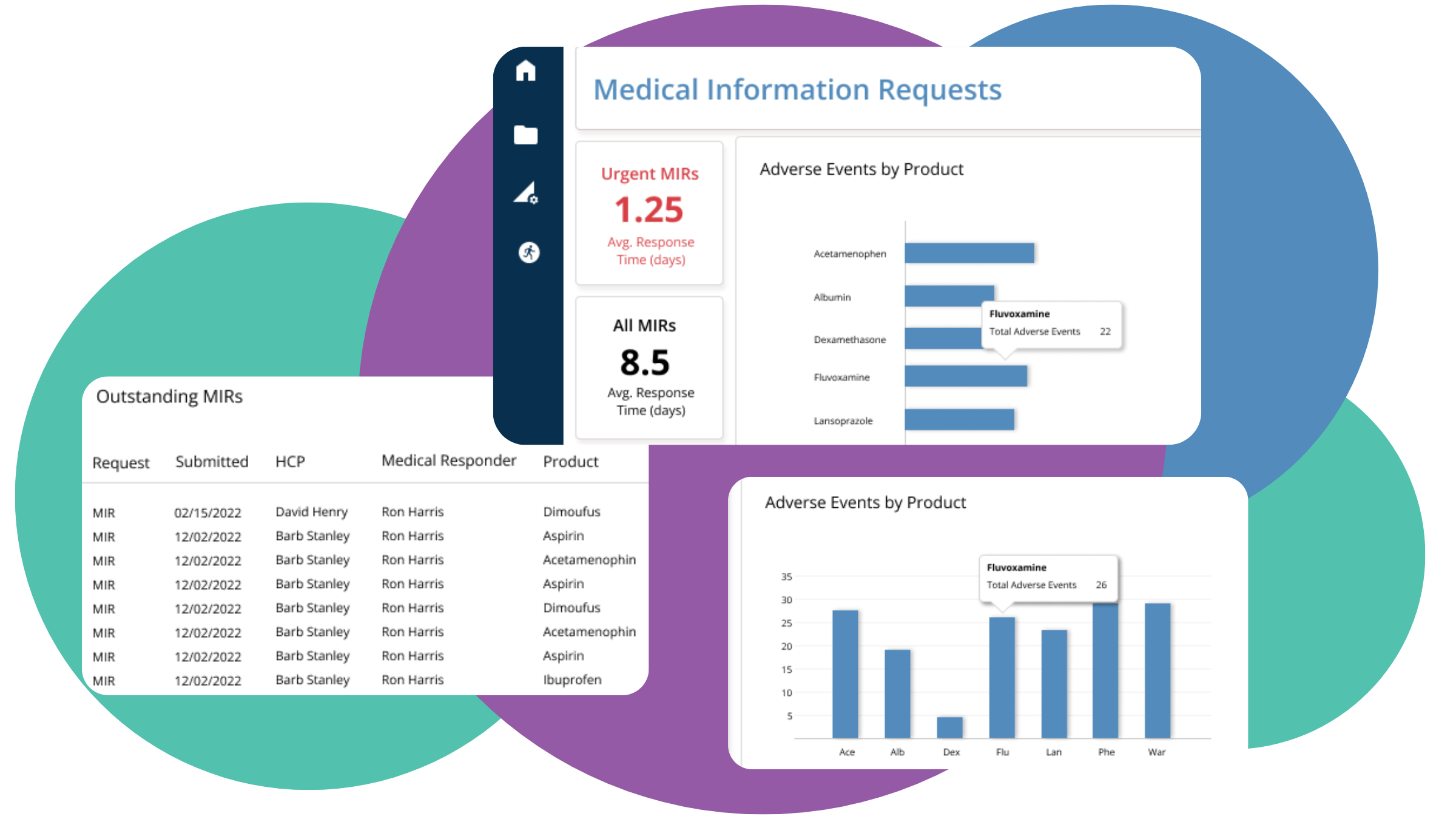
Our advanced platform also enables seamless integration with existing systems and workflows, making it easy to adopt and implement into your existing infrastructure.
With BP Logix's MIR solution, you can ensure that your organization is equipped to manage MIRs effectively, promote compliance, and protect your reputation in the healthcare industry. Contact us today to learn more and take the first step towards optimizing your MIR management process.