An adverse event report (AER) is a self-initiated medical report detailing an undesirable clinical outcome associated with a medication or medical device. The reports fall under the umbrella of medical information requests (MIRs), as both are initiated by providers or consumers and are then directed at pharmaceutical companies or medical device manufacturers.
AERs are typically generated by physicians, though manufacturers or consumers may also submit them. Regardless, adverse event reporting is critical to patient safety and the refinement of medications. Without the information provided by AERs, manufacturers would be unable to track the frequency of unfavorable events or monitor the severity of unintended clinical outcomes.
By leveraging adverse event reports, manufacturers can continuously improve their products, protect consumers, and remove potentially dangerous medications from circulation. However, although virtually every entity in the life science and healthcare industry understands the importance of adverse event reporting, many struggle to efficiently manage their AERs.
As part of our efforts to expedite adverse event reporting, we have identified some common forms of adverse events, outlined the challenges associated with their AER submission, and provided some strategies to circumvent these roadblocks. Care providers and other entities in the life science and healthcare sectors can use the insights below to promote compliance and expedite reporting.
Types of adverse events
Any unintended and undesirable clinical outcome caused by an administered medication can be categorized as an adverse event. Some of the adverse events that warrant an AER include the following:
-
Unexpected or harmful side effects brought on by drugs or medical devices
-
Overdoses or accidental exposure to unnecessary medications
-
Issues related to product quality, purity, or potency
-
Cases of medical errors or problems related to product labeling or packaging
-
Environmental or occupational exposure to chemicals or other substances
-
Incidents that fall into the above categories should be classified and documented via an adverse event report in most instances.
Failing to file an AER promptly and efficiently can expose a provider and their organization to significant liability while endangering patients and hindering manufacturers’ ability to improve their offered medications or devices.
Reporting unexpected or harmful side effects is particularly important and time-sensitive. Such occurrences must be reported as soon as possible to relay the information to the Food and Drug Administration (FDA) and the product manufacturer.
When filing reports, providers must differentiate between side effects that were explicitly harmful to the patient and those that were simply unexpected. For instance, suppose a provider prescribes a new headache medication to some patients. One patient reports nausea and vomiting, whereas another finds the drug improved their sleep quality.
While the pharmaceutical company needs to know about each incident, reports are documented using distinct processes. Therefore, both outcomes should be properly reported; the negative effect through an AER and the positive result via a medical information request form.
The challenges of adverse event reporting
Adverse event reporting is a relatively straightforward process: Providers should promptly report any undesirable clinical outcome caused by a medication or device. Despite its relative simplicity, however, the reporting process is muddled due to a wide range of confounding factors, including the following:
Regulatory compliance
Adverse event reporting is a tightly regulated process, and companies that fail to adhere to these strict ordinances can incur hefty penalties and lawsuits. Additionally, non-compliant organizations will likely suffer long-lasting reputational damage, which could negatively impact consumer trust for years.
Unfortunately, a single governing body does not set adverse event reporting regulations. While the FDA does establish many AER protocols, companies must also contend with their state-specific regulations. Navigating a single set of regulatory guidelines can be challenging enough, so keeping up with multiple sets of continuously evolving requirements is undoubtedly even more difficult.
Data silos
Far too often, adverse event data is stored in multiple systems across different departments. When data is scattered and stored on disparate software solutions that don’t properly communicate, the details surrounding adverse events will get lost in the shuffle.
Data silos hinder the proper documentation of AERs and make it challenging to submit or manage any medical information requests. Organizations in such scenarios often struggle to track and analyze adverse event information, much less report it within established time constraints.
Therefore, businesses must proactively work to eliminate data silos while maintaining their AER and MIR compliance. Doing so will also promote better organizational efficiency and improved collaboration among team members.
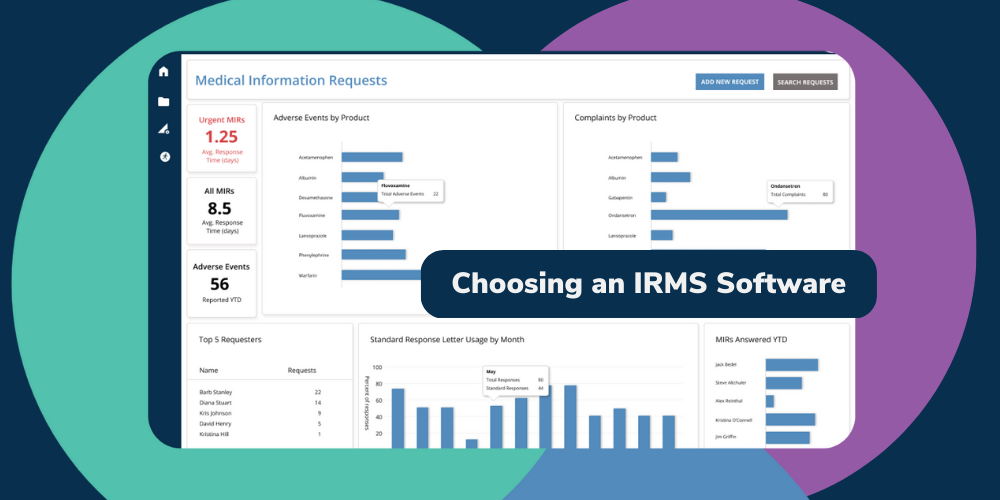
Adopting a modern, fully-integrated reporting solution is the most practical way to tear data silos down. A platform like BP Logix’s MIR solution facilitates the seamless tracking and analysis of all outstanding medical information requests, including adverse event reports. Organizations can ensure they meet regulatory requirements and preserve their reputations.
Managing large volumes of data
Adverse events include a broad array of harmful and unintended clinical outcomes. As a result, organizations are bound to rack up a large volume of AERs, and keeping up with that data can prove quite challenging.
Entities not adopting streamlined data management workflows will struggle to respond to AERs within established time constraints, leaving the door open to compliance violations and penalties.
As part of their data management protocols, organizations must also be able to categorize and prioritize their AERs. More significant adverse events, such as hospitalization, need to be addressed before less pressing ones.
Responses must be timely
Delays in adverse event reporting can be highly detrimental to an institution’s reputation. More importantly, they can pose a serious risk to patients. The longer potentially dangerous or compromised batches of medication remain in circulation, the higher the chances are for a severe event, such as permanent injury or death. Even a single negligence-related death or injury can expose an organization to significant legal repercussions.
For instance, let’s say that a physician’s patient is hospitalized due to a severe reaction to a medication. The physician does their due diligence, immediately submitting an AER to the manufacturer, but that manufacturer does not have a means of prioritizing its AERs. Instead, it processes them in the order they were received, which means the original AER is not responded to for upwards of 30 days. During that time, two dozen other patients are hospitalized.
The above example might seem somewhat hyperbolic, but it illustrates the dangers of adverse event reporting delays in that they magnify an organization’s risk. Therefore, life science entities must make every effort to expedite their reporting and response processes.
Complex processes
Generating and responding to AERs can be an incredibly involved, complex process. Even a single AER may require input from multiple stakeholders, such as regulatory bodies, internal teams, and healthcare providers. The process becomes even more complicated when patients are directly involved in adverse event reporting.
While there is no way to remove stakeholders from the equation, organizations can facilitate better collaboration and information sharing by breaking down communication barriers between these entities. When stakeholders can efficiently provide and relay their insights, the reporting process becomes streamlined.
How to overcome these challenges
Adverse event reporting is an intricate procedure that involves dealing with several different hurdles. Still, although there is no singular solution for overcoming these challenges, organizations can simplify and expedite their AER processes by proactively eliminating points of friction.
Specifically, companies striving to facilitate more efficient adverse event reporting should do the following:
Establish clear internal procedures and guidelines.
Though they may seem obvious, some organizations do not have clear, repeatable processes for adverse event reporting. When reporting guidelines are unclear and easy to follow, errors are bound to occur.
Any organization interested in revamping its AER management strategies should reevaluate its internal procedures and guidelines, gathering feedback from employees that submit or receive AERs. Doing so will help stakeholders make meaningful improvements to existing protocols.
Use a centralized adverse event reporting system to eliminate silos/improve visibility
Adverse event reporting will always be an organizational pain point if data silos exist. In light of such, organizations must replace their antiquated, disparate reporting systems with a centralized alternative. Aggregating all adverse event reports into a single platform allows businesses responsible for processing or responding to AERs to promote better visibility and improve compliance.
Adopting a unified database will improve AER visibility, eliminate data silos, and promote enhanced medical information request management. Addressing MIR and AER management is critical to compliance within the life science and healthcare verticals. An organization that succeeds at both will be able to foster trust among its consumers and providers while also avoiding any regulatory penalties.
Implement process automation to reduce errors
Deploying a centralized AER management database is a significant step toward better efficiency and compliance. Still, if adverse event reporting workflows continue to rely on manual inputs, they will always be plagued by human errors. Organizations tasked with AER management should implement process automation tools like BP Logix’s MIR solution to combat errors and further mitigate risks.
Our dynamic platform enables entities to automate traditionally tedious, manual processes, in turn freeing up AER management teams to tackle more dynamic tasks while simultaneously reducing the frequency of errors and mitigating their risks. BP Logix’s MIR solution integrates into an organization’s existing reporting system and can be tailored to meet the needs of nearly any healthcare or life sciences entity.
Leverage predictive analytics to identify trends
When AERs are dispersed across an entire team, detecting and tracking trends among them can be tricky. The longer a concerning pattern of adverse events goes undetected, the more likely it is to result in reputational damage and patient harm.
With that in mind, companies must incorporate data analytics software into their AER management workflows. Analytics and machine learning tools can detect trends as soon as they emerge, allowing stakeholders to use real-time data to guide decision-making processes.
Provide training and support to employees
Although workflow automation technologies can improve reporting efficiency, employees will always be the backbone of any AER initiative. Adverse event reporting will remain a challenge without talented, well-trained, confident workers.
Therefore, decision-makers must invest in employee training and upskilling efforts to turn AER management into an organizational strength, all of which must be designed with the employee in mind.
Generally speaking, it is best to avoid long, tedious training blocks and break down programs into smaller, easier-to-digest sessions. Such an approach can improve information retention and help team members get the most out of each training session.
Ensure compliance with regulatory requirements with regular audits
The abovementioned strategies should significantly improve an organization’s adverse event reporting processes. Still, organizational leaders must ensure compliance with regulatory requirements by conducting regular audits and process reviews. These reviews will reveal which tactics are working and what shortcomings still exist within the AER management strategy of the business.
If possible, organizational leaders should outsource audits to ensure objectivity. Using a third-party auditing firm can also provide additional insights, as the outside source may be able to offer a fresh perspective on old business challenges.
Modernize adverse event reporting
While many challenges are associated with adverse event reporting, your organization must navigate each to ensure compliance and preserve its reputation within the healthcare community.
 You can create a dynamic strategy to facilitate streamlined reporting by leveraging the adverse event reporting tactics outlined above. When you pair that strategy with BP Logix’s robust MIR management solution, you can further expedite your reporting processes and achieve ongoing compliance.
You can create a dynamic strategy to facilitate streamlined reporting by leveraging the adverse event reporting tactics outlined above. When you pair that strategy with BP Logix’s robust MIR management solution, you can further expedite your reporting processes and achieve ongoing compliance.
BP Logix’s platform includes a suite of automation and visibility tools designed to help you track MIRs and adverse events throughout the reporting process. You can prioritize time-sensitive requests and avoid reporting mishaps that expose your business to fines or other penalties. Additionally, our solution reduces instances of human error and facilitates complete reporting of adverse events.
If you are ready to rethink how you manage adverse event reporting, explore BP Logix’s MIR solution to learn more.