Get full control of regulatory affairs with the workflow automation tool that's easy to use, configurable to your processes, and affordable.
Regulatory submissions are not managed together.
Centralize the processes with Approvia.
Structure your FDA submission, then let Approvia handle automated review assignments and focus tasks across multiple departments.
Approvia is designed to be customized to your standard operating procedures, which prevents your users from adopting clunky workarounds or switching between multiple applications.
Our novel approach to review and approval assignments allows your external stakeholders to securely contribute to documents, without the need for a login or password.
Approvia's granular audit logs ensure your application and regulatory submission documents follow FDA guidelines, eliminating the risk of delays or rejection.
We configure PubPro to your unique SOPs, which prevents your users from adopting clunky workarounds or switching between multiple applications.
PubPro ensures your publication management processes are air-tight and constantly adapting to ever-changing regulatory and business requirements.
Approvia offers the perfect balance of pre-built functionality and flexibility, with 80% of the solution already built and the remaining 20% configured to your unique requirements.
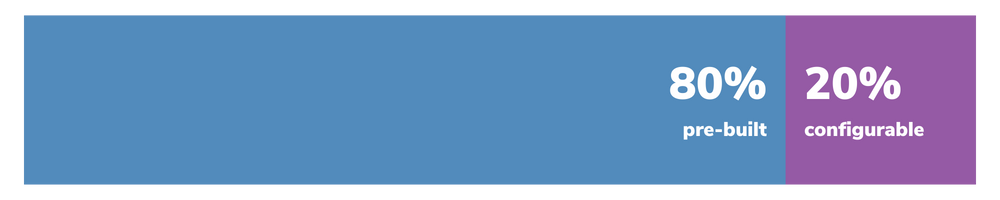
This means you get the speed and affordability you need, plus powerful customization options not available in competing regulatory affairs management software.
Our flexible, scalable process automation solutions empower medical affairs teams at life science organizations to operate better and do their best possible work.

With Process Director, you get a process engine that works every time.
There's endless possibilities for automating and optimizing other processes, whether you use one of our pre-built use cases or create your own.
Enjoy the greatest bang for your buck as you create more new processes.
Get the convenience of SaaS with the added luxury of a consultative business partnership. We’re there to support you every step of the way.